Through process optimisation and lower costs during development and production of viral vectors, we contribute to the innovation and accessibility of Cell & Gene Therapies (CGT) globally.
Our suspension based industry recognized viral vector process platform serves the needs for scalability in manufacturing.
The team continues to work on optimizing plasmid constructs and solutions on other technology engineering challenges to provide be-spoke options to clients.

SGVector has established an efficient, scalable and reproducible production process and analytical platform that is able to produce viral vectors with high yield and achieve safety, purity and quality attributes that meet regulatory requirements.
Design and develop functional viral vectors including design of Rep-Cap helper and transfer plasmids, optimisation of transgene expression to enhance vector functionality for research and clinical applications
Optimize the process from small scale to large-scale including cell expansion, transfection, clarification, purification, polishing, formulation, filling and production (manufacturing)
Provide assay development and testing to ensure the quality, purity, and potency of the viral vector product
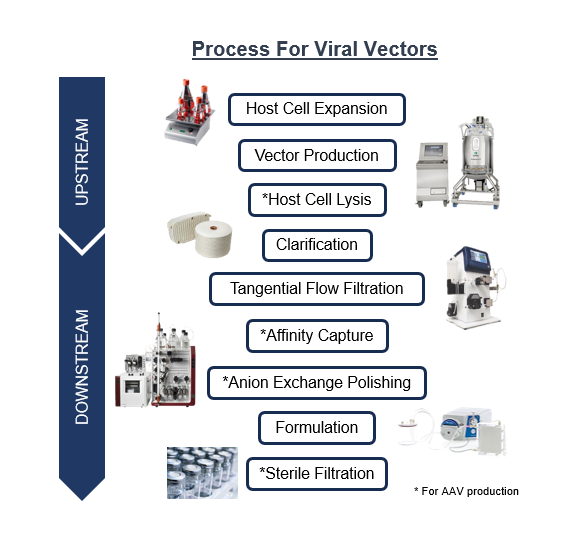
Gene therapy aims to introduce a new or corrected gene into cells to replace or compensate for a defective one. Viral vectors are modified viruses that can enter cells and deliver the therapeutic genes. Viral vectors are commonly used because of their ability to efficiently and specifically target certain types of cells and tissues.
Viral vectors are designed to be safe and to minimize the risk of adverse side effects. Viral vectors are an essential tool in gene therapy, allowing for the efficient and targeted delivery of therapeutic genes to specific cells and tissues in the body.
In the recent years, AAV gene therapy has emerged as a groundbreaking tool that demonstrated immense potential for treating various genetic and acquired diseases with long lasting therapeutic effects. With over 200 FDA approved clinical trials involving AAVs and the rapid development of AAV based gene therapies, it has resulted in an urgent need for a scalable manufacturing platform to meet the increasing demand for recombinant AAVs.

Our 250-square meter facility is fully equiped for the design and development needs of viral vectors and this includes but not limited to process development, its optimisaion, assay development, analytical and safety testing as well as warehousing for raw materials, plasmids, MCB and the finish products.
The range of modern equipment in the facility includes bioreactors up to 50L, chromatography system, TFF System, dPCR and qPCR systems, to name a few.
At SGVector, we are committed to accelerating your drug discovery and development programs.
Whether you are a pharmaceutical company, biotechnology firm, or academic institution, we are here to be your trusted partner in achieving your research and development goals.
Contact us today to discuss your project requirements and learn how SGVector can contribute to your success.
Let us be your partner in advancing science and improving global healthcare.
Your provider of choice for viral vector development & manufucturing for cell & gene therapies.
Get the latest updates via email. You may unsubscribe anytime.